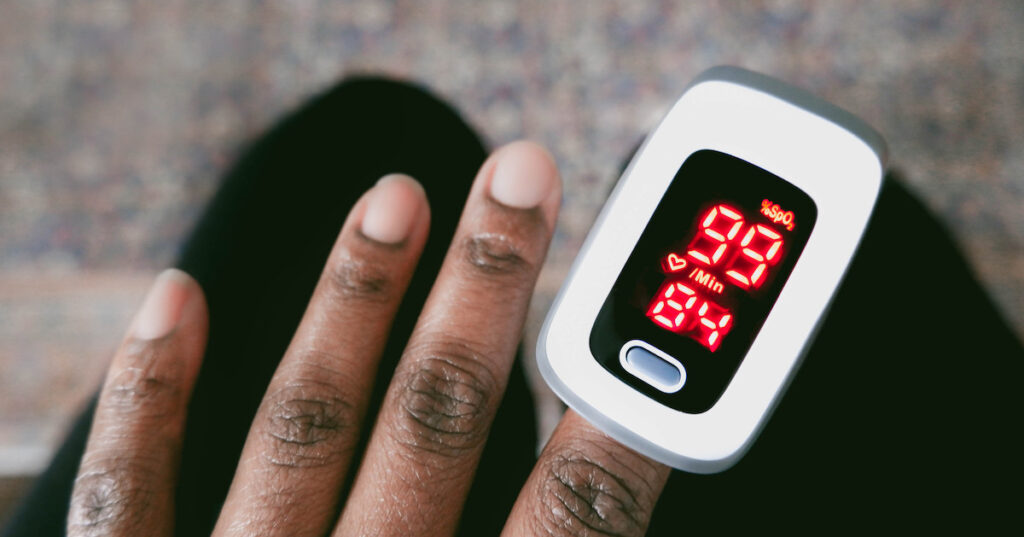
The U.S. Food and Drug Administration has published updated draft recommendations to help improve the performance of pulse oximeters across skin tones.
Posted inUncategorized

The U.S. Food and Drug Administration has published updated draft recommendations to help improve the performance of pulse oximeters across skin tones.